A chemical probe molecule—a “first gen” molecule that can be used for drug development—that targets a host cell’s protein quality control pathways can dramatically reduce infection by Dengue and Zika viruses. The research led by Lars Plate, assistant professor of chemistry and biological sciences, is a significant step toward host-directed antiviral therapeutics that act on host cells and not the virus itself.

The article, “Small molecule endoplasmic reticulum proteostasis regulator acts as a broad-spectrum inhibitor of Dengue and Zika virus infections,” was published in the journal Proceedings of the National Academy of Sciences on Jan. 13.
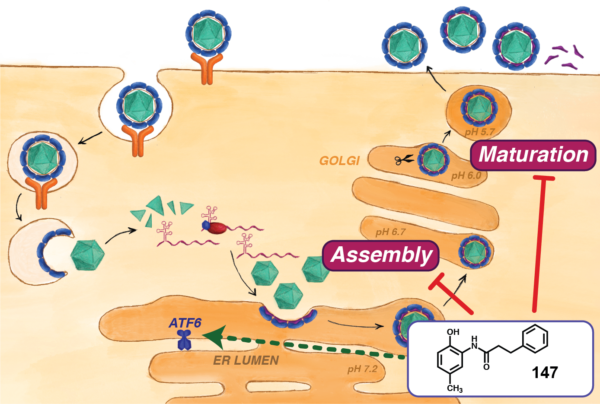
One-third of all proteins—a major biomolecule in cells and viruses—are folded into three-dimensional, origami-like structures, and they replicate within an organelle called the endoplasmic reticulum. Plate’s current interest is in the host cell’s protein pathways, with the goal of keeping viruses from hijacking them to fold their own proteins.
Led by graduate student Katherine Almasy, Plate’s team demonstrated that a molecule called 147 inhibits Zika and Dengue virus replication within human cells. Healthy proteins and cells behave like a lock and key, with proteins binding exactly to the next cell in virus replication. 147 targets five to 10 different host proteins, some of which are important for viral replication. The molecule changes the shape of the protein “key” so it can no longer fit in the host cell “lock”—inhibiting viral replication while not affecting enough of the protein to harm the host cell. The researchers concluded that 147 is up to 95 percent effective in reducing virus replication.
147’s efficacy represents a promising new way of helping block the spread of many viruses when used in concert with other therapeutics. “If we can use 147 as one piece of a combination therapy, we may see additive effects,” Plate said. “We may also be able to get around the problem of drug resistance that plagues many therapies if a virus mutates too quickly.”
Plate deems the discovery serendipitous and directed his team’s efforts to understand how 147 works. The researchers saw that while the molecule did not reduce the number of viral particles produced, the viruses that were created were no longer infectious. “The molecule should have made protein folding better, but instead it disrupted how well the virus could enter the next host cell,” Plate said. “The intricacies of the mechanism of action were a very surprising discovery.”
Plate and his group are setting out to understand exactly how the protein shape is changed. Many other virus families, including SARS-CoV-2 and coronaviruses, take advantage of ER protein quality control during infection, making it interesting to explore whether the strategy can be broadly applied.
The research was funded by the National Institute of General Medical Sciences grant R35GM133552 and Vanderbilt University startup funds.