Medical cannabis for opioid use disorder: The association between state policies and dispensary marketing claims
Cannabis might have benefits for opioid use disorder treatment but it has yet to be FDA-approved for treatment in the United States due to a lack of rigorous research. Nonetheless, several states designate opioid use disorder as a qualifying condition for receiving legal access to medical cannabis. These policies could cause cannabis dispensaries to market medical cannabis as an official treatment for opioid use disorder. Without the science to back them up, these claims could ultimately harm individuals with opioid use disorder. This study assessed whether there was a relationship between state medical cannabis policies and cannabis industry marketing claims. Findings revealed a greater prevalence of unconfirmed claims (e.g., cannabis can be used as an alternative to opioid use disorder medications) among dispensaries located in states where opioid use disorder has been designated as a qualifying condition for medical cannabis.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
The cannabis industry has often marketed cannabis with an emphasis on its therapeutic benefits for various medical conditions. One such claim is that cannabis can help treat opioid use disorder (OUD), with some claiming that cannabis can be used in place of FDA-approved medications (e.g., buprenorphine, methadone). However, there is currently a lack of rigorous scientific evidence to support these claims and unconfirmed claims have important implications for treatment seeking and overdose. For example, medications like buprenorphine and methadone are proven to help prevent opioid-related overdose and individuals with OUD who forgo these medications have significantly increased risk of mortality. Therefore, encouraging patients to seek cannabis as an alternative to medication treatment could ultimately hinder efforts to reduce overdose and related deaths.
Despite ongoing need for randomized controlled trials to determine cannabis’ true therapeutic benefits and risks, several states’ medical cannabis policies designate OUD as a qualifying condition for legally receiving medical cannabis.
These policies might give dispensaries implicit permission to market medical cannabis as a treatment for OUD, which could ultimately harm individuals with OUD given that the therapeutic effects of cannabis for OUD have not been appropriately tested.
The current study assessed the association between medical cannabis policies and therapeutic claims made by the cannabis industry by comparing the marketing claims of dispensaries located in states where OUD is a designated as a qualifying condition for medical cannabis to the marketing claims of dispensaries in states where it is not a qualifying condition.
HOW WAS THIS STUDY CONDUCTED?
The authors conducted a cross-sectional mixed methods study of website and social media content among 167 medical dispensaries in 7 states. Dispensaries were identified via state Department of Public Health and industry websites. Three states had OUD designated as a qualifying condition for their medical cannabis programs, including New Jersey, New York, and Pennsylvania, which collectively had a total of 44 dispensaries. The authors also identified 123 dispensaries located in four nearby states (i.e. Connecticut, Delaware, Maryland, and Ohio) that did not have OUD as a qualifying condition for medical cannabis. Dispensary websites and social media content were each assessed by two researchers, for mention of six key words: (1) opioid, (2) opioids, (3) addiction, (4) methadone, (5) buprenorphine, (6) suboxone. Text mentioning key terms was recorded and subsequently re-categorized by three independent researchers into four binary (i.e. yes/no) categories: (1) Dispensary claimed cannabis can treat OUD, (2) dispensary claimed cannabis can replace FDA-approved medications for OUD (i.e. buprenorphine, methadone, naltrexone), (3) dispensary claimed cannabis can be an add-on treatment (adjunct) to FDA-approved medications for OUD, or (4) dispensary claimed cannabis can be a substitute for opioids to treat other conditions like chronic pain. The authors then calculated the percentage of dispensaries that mentioned opioids, and the percentage of dispensaries falling within each of the four binary categories and examined differences between states with and without policies designating OUD as a qualifying condition for medical cannabis.
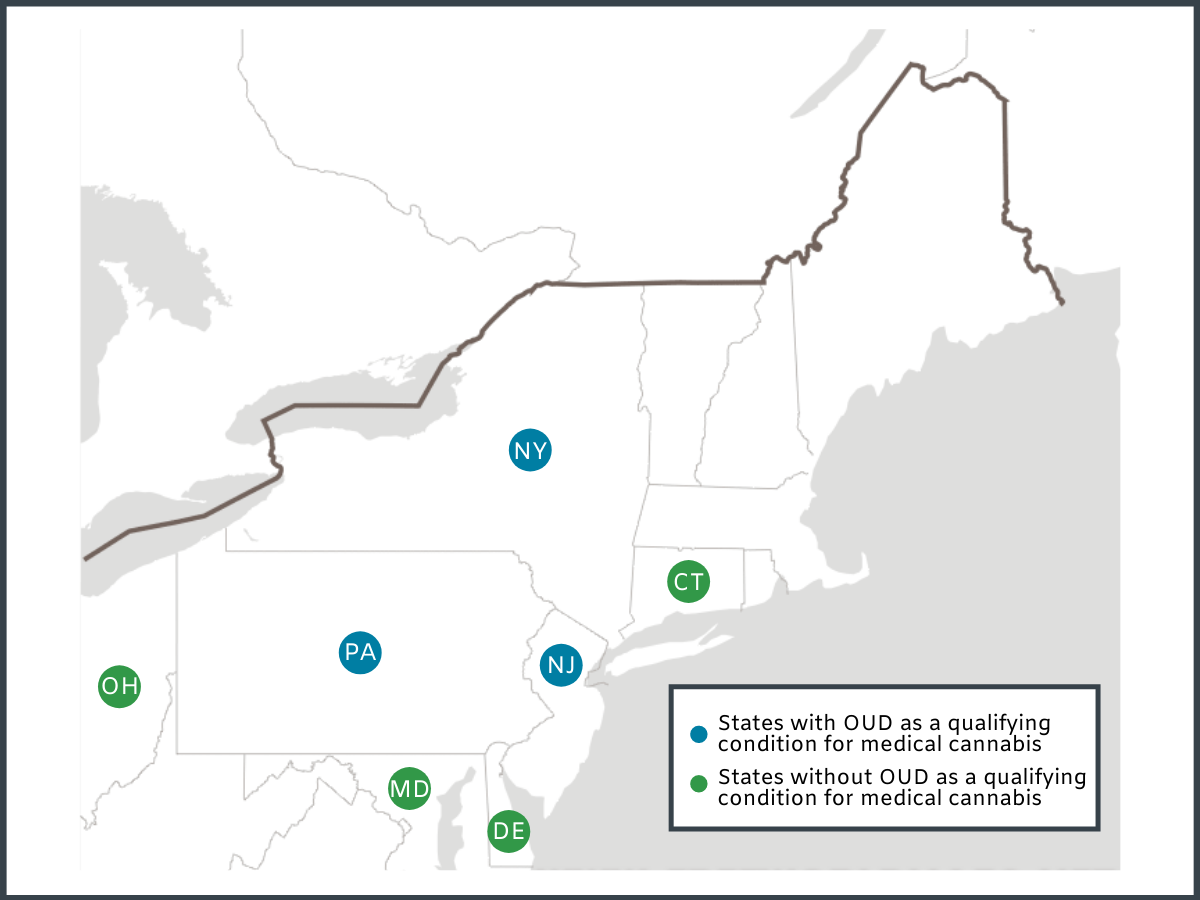
Figure 1.
WHAT DID THIS STUDY FIND?
Most dispensaries mentioned opioids in website and media content, but mentions were more prevalent in states where OUD was a qualifying condition for medical cannabis.
Opioids were mentioned in website and media content by 86% of dispensaries in states with OUD as a qualifying condition, and by 60% of dispensaries in states without OUD as a qualifying condition.
All four opioid-related medical cannabis claims were more prevalent among dispensaries located in states where OUD was a qualifying condition for medical cannabis.
Compared to dispensaries in states where medical cannabis has not been approved for OUD, dispensaries in states with OUD approved medical cannabis policies were more likely to claim that cannabis can treat OUD (65% vs. 26%), replace FDA-approved medications for OUD (18% vs. 4%), be an adjunct to FDA-approved medications (30% vs. 2%), and function as a substitute for opioids to treat other conditions, like chronic pain (72% vs. 47%).
When considering cannabis company brands, eleven brands (~7% of all brands assessed) operated in both policy exposed and policy unexposed states, and marketing claims made by these companies did not differ by state, suggesting that the brand is important to consider when evaluating policy-marketing relationships.
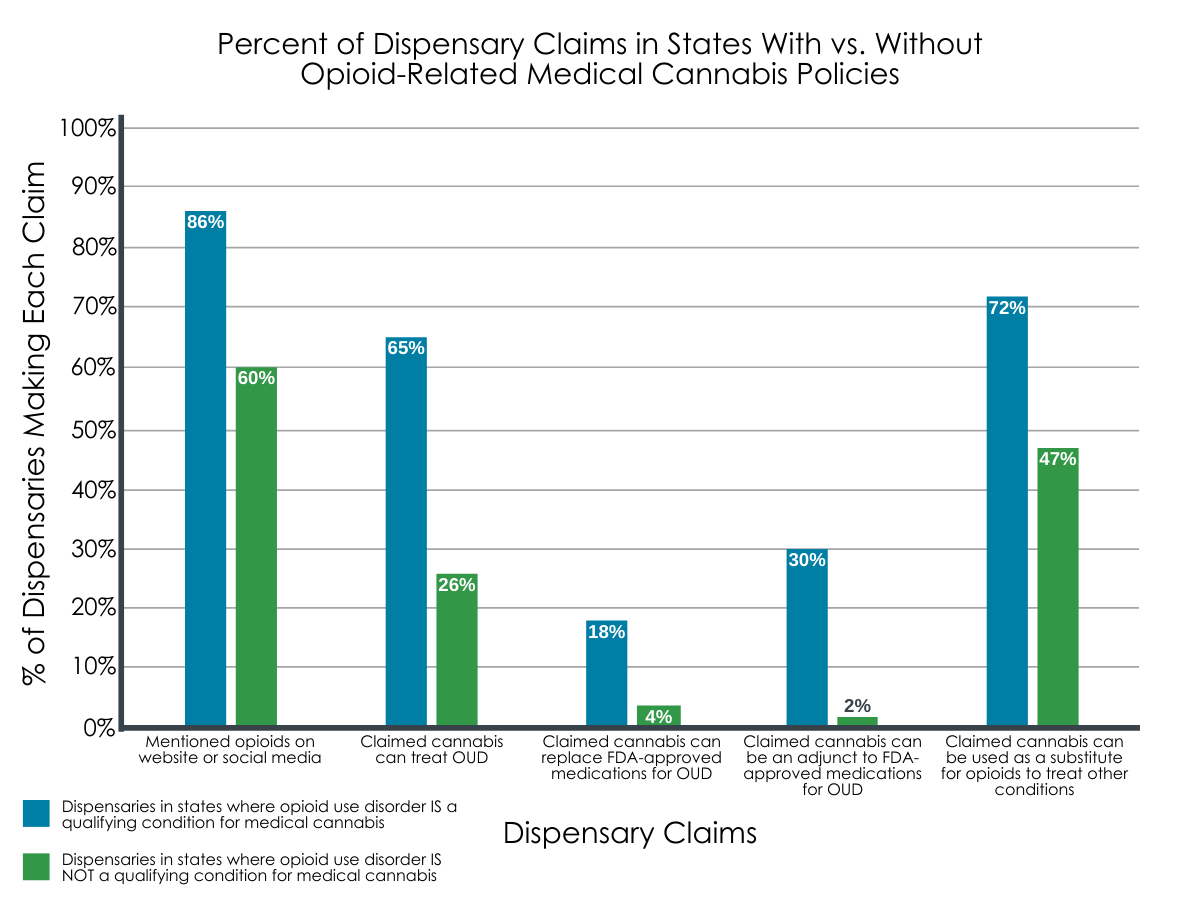
Figure 2. Graph above depicts the percentage of dispensaries that made each marketing claim in states with and without opioid use disorder (OUD) designated as a qualifying condition for medical cannabis. In all cases, dispensaries’ opioid-related claims were more prominent among those located in states where medical cannabis was approved for OUD.
Excerpts from Dispensaries’ website and social media content in support of medical cannabis for OUD:
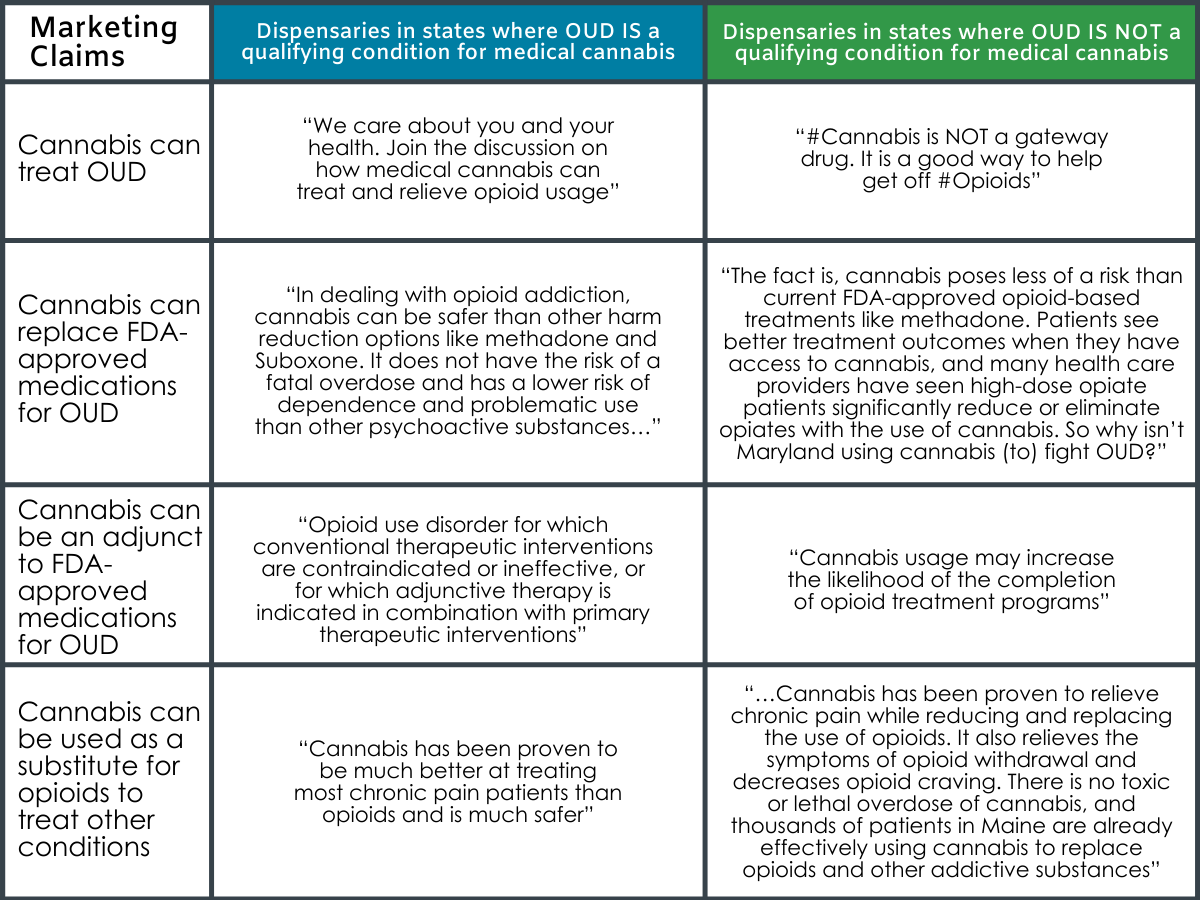
Figure 3.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
Despite lack of FDA-approval, and little rigorous data to show benefit, some U.S. states have passed policies designating OUD as a qualifying condition for medical cannabis. The current study suggests that these policies might promote unsupported claims by the cannabis industry about cannabis’ benefits for OUD treatment.
Compared to dispensaries in states without such designations in their legislation, a greater percentage of dispensaries in states with cannabis-approved treatment policies made marketing claims that cannabis can treat OUD, replace FDA-approved medications for OUD (e.g., buprenorphine), and can be used as an add-on treatment to FDA-approved medications and as a substitute for opioids to treat other conditions like pain.
Importantly, these findings speak to the relationship between policies and marketing, rather than the direct effects of legislation on marketing claims. Therefore, it is unclear whether the policies influenced marketing, or if unsupported marketing claims influenced legislative changes.
Although more frequent in states where medical cannabis is approved for OUD, any mention of opioids on dispensary websites and social media posts was relatively common across all states. Therefore, it is important to evaluate how dispensaries’ marketing content influences patients’ decision making when they are considering different treatment pathways, and how this relates to recovery outcomes and overdose rates.
A large literature has revealed the influence of general product-marketing on consumer and patient behaviors. Research looking at cannabis industry marketing suggests it influences consumer behavior among other populations; for example, advertisements have been associated with increased cannabis use among adolescents. In the context of the current study, unconfirmed claims made by the cannabis industry might therefore mislead patients to seek cannabis as a treatment for OUD and/or forgo evidence-based treatments like methadone and buprenorphine (i.e. opioid agonist treatments). Given their ability to help prevent opioid-related overdose, individuals who forgo these medications might have an increased risk of mortality. Opioid agonist treatments are more effective for overdose prevention than abstinence and opioid antagonist treatments like naltrexone. Therefore, encouraging patients to seek cannabis as an alternative to medication treatment, without fully understanding its therapeutic risks and benefits, could ultimately put individuals at higher risk for overdose.
Findings to date regarding overdose and medical cannabis are mixed – early studies suggested that state laws approving of medical cannabis for OUD were associated with reduced opioid overdose in those states, but more recent studies found the opposite (i.e. associated with increased overdose). Nonetheless, studies looking at relationships between variables do not speak to the direct effects of medical cannabis and are not sufficient for obtaining FDA-approval for cannabis as an OUD medication.
Claims that cannabis can be used as an adjunct to OUD medications are also cause for concern. Cannabis can increase buprenorphine’s and methadone’s availability in the body, leading to toxicity at higher doses, and also has the potential to hinder medication treatment outcomes. Some studies suggest cannabis use might increase risk of illicit opioid use and treatment dropout in buprenorphine and methadone patients. However, other studies contradict these findings and suggest cannabis can benefit treatment (e.g., higher rates of retention in methadone and buprenorphine patients after 6 months of treatment). Additional research is clearly needed to understand conflicting reports and assess the influence of dose, different cannabis strains, and different OUD cohorts.
In sum, the use of cannabis as a treatment for OUD has largely outpaced the scientific evidence backing it. Recent research suggests that cannabis has the potential to reduce withdrawal symptoms and prevent opioid misuse and relapse, but this literature is also incomplete, and outcomes thus far are mixed. As with any area of medicine, high-quality clinical research is needed to confirm preliminary findings and to systematically investigate the risks associated with medical cannabis for OUD treatment. Identifying alternatives to medication treatment and abstinence might help address relatively low treatment seeking rates, as they might appeal to individuals who prefer opioid-free treatment pathways and to those who experience barriers to pharmacotherapy access or untoward medication side effects. Until rigorous research is conducted to fully understand medical cannabis’ potential as an OUD treatment, states should be on the lookout for unconfirmed claims made by their local dispensaries and might consider novel policies that better police unsupported claims and/or further educate the cannabis industry and community about evidence-based treatments for OUD and the science backing them. Until the scientific evidence is sufficient enough to form conclusions, legislators should heed caution in designating OUD as a qualifying condition for medical cannabis.
- LIMITATIONS
-
- This study examined seven states and additional research is needed to determine if marketing material in other states with similar legislature yields similar results. The effects of marketing on consumer and patient behaviors was also not evaluated and it is therefore unclear if claims made by dispensaries have a direct influence on treatment-related decision making.
- Investigation was limited to cannabis dispensaries with online content (website and/or social media accounts). Therefore, marketing materials inside of store-front businesses and businesses without online content were not evaluated, and additional research is needed to determine if claims differ in these dispensary settings.
- These data are not longitudinal, which limits our ability to determine if the dispensary claims occurred as a direct result of the state policy, or if dispensary claims about medical cannabis’ therapeutic benefit were made before these policies were implemented.
BOTTOM LINE
- For individuals and families seeking recovery: The potential for cannabis as a treatment is not confirmed by rigorous research (i.e. randomized controlled studies, which are necessary for FDA approval of drug treatment) and the effects of cannabis on opioid overdose and treatment outcomes are not yet clear. Given the lack of scientific evidence to date, individuals and families seeking recovery should be cautious when interpreting cannabis dispensary claims and are encouraged to consult with their physician to ensure the right treatment option for them.
- For treatment professionals and treatment systems: Due to the lack of rigorous evidence to date, treatment providers should remain cautious about recommending cannabis for OUD treatment and are encouraged to educate patients on this growing literature to ensure informed treatment-related decision making.
- For scientists: With a lack of rigorous research conducted to date, the use of cannabis as a treatment for OUD has outpaced the scientific evidence backing it. Randomized controlled trials on cannabis for OUD will provide key information regarding its efficacy, which can then inform practice and policy. Further research examining the effects of cannabis on overdose and treatment/recovery outcomes, as well as the effects of opioid-focused dispensary marketing on patient behaviors (e.g., treatment decisions) will help elucidate the benefits/risks of cannabis and industry marketing among individuals with OUD.
- For policy makers: In an effort to protect individuals with OUD, state legislators should heed caution in designating OUD as a qualifying condition for medical cannabis until scientific evidence in safety and efficacy is established. Given that opioid-based medications (e.g., buprenorphine, methadone) have been proven to be one of the only things to reduce opioid overdose deaths, state legislators might also consider implementing policies that better regulate cannabis marketing content, as well as state programs that educate the cannabis industry about the potential harms of unsubstantiated claims.
CITATIONS
Shover, C. L., Vest, N. A., Chen, D., Stueber, A., Falasinnu, T. O., Hah, J. M., … & Humphreys, K. (2020). Association of state policies allowing medical cannabis for opioid use disorder with dispensary marketing for this indication. JAMA Network Open, 3(7), e2010001. doi: 10.1001/jamanetworkopen.2020.10001

