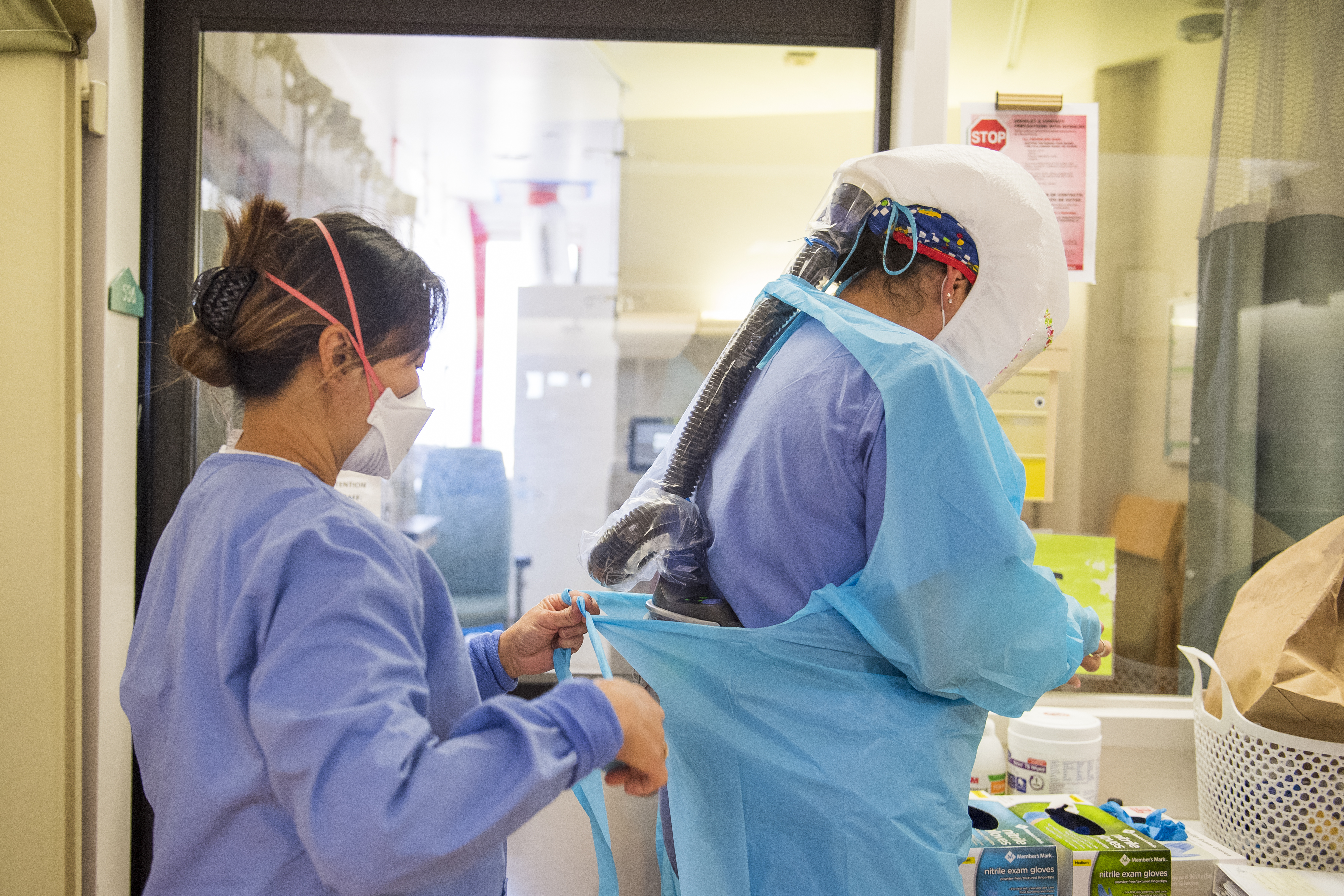
FILE – In this March 29, 2021 file photo, a worker readies syringes with the Moderna COVID-19 vaccine in Metairie, La. The Centers for Disease Control and Prevention is urging all pregnant women to get vaccinated against COVID-19, Wednesday, Aug. 11. The advice comes as hospitals in hot spots around the U.S. see disturbing numbers of unvaccinated mothers-to-be seriously ill with the virus.
So far, Americans who received the Moderna COVID-19 vaccine can't get booster shots, but could that soon be changing?
Bloomberg, citing people familiar with the matter, reported last week that the U.S. Food and Drug Administration was leaning toward authorizing half-dose booster shots for those who received Moderna's two-shot mRNA vaccine.
Stream NBC10 Boston news for free, 24/7, wherever you are.
That would mark the second COVID-19 vaccine to get authorization from the FDA for booster doses.
U.S. health officials already recommended boosters of the Pfizer and BioNTech vaccine for some people at higher risk for severe illness from COVID-19 based on evidence that protection against milder disease can wane, especially among older adults.
Get updates on what's happening in Boston to your inbox with our News Headlines newsletter.
Here's what we know so far:
Can I get a booster shot now if I got the Moderna vaccine?
So far, the FDA has only approved Pfizer boosters, to be administered at least six months after completion of the primary series in:
- individuals 65 years of age and older
- individuals 18 through 64 years of age at high risk of severe COVID-19
- individuals 18 through 64 years of age whose frequent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19 including severe COVID-19.
U.S. regulators are expected to decide at a later date on widespread boosters for COVID vaccines by both Moderna and Johnson & Johnson, however.
Moderna submitted data to the FDA seeking evaluation for its booster shot on Sept. 1.
“We are pleased to initiate the submission process for our booster candidate at the 50 µg dose with the FDA. Our submission is supported by data generated with the 50 µg dose of our COVID-19 vaccine, which shows robust antibody responses against the Delta variant,” Stéphane Bancel, Moderna's CEO, said in a statement.
But as the FDA's Vaccines and Related Biological Products Advisory Committee debated administering third doses of Pfizer and BioNTech's vaccine this month, federal health regulators said they needed more time to review Moderna's application for extra doses.
Adding to the complexity of further decisions on booster shots, Moderna wants its third dose to be half of the original shots.
Moderna previously released data on breakthrough cases, saying it supports the push for wide use of COVID-19 vaccine booster shots.
When might I be able to get a booster shot?
Despite the resistance in recent days, some top U.S. health officials said they expect boosters to eventually win broader approval in the coming weeks or months. Dr. Anthony Fauci said recently that “this is not the end of the story.”
The CDC panel stressed that its recommendations will be changed if new evidence shows more people need a booster.
Further timing remains unclear.
Fauci said last week that safety and efficacy data on mixing and matching COVID vaccines with boosters could be available within the next two weeks.
"As with all things we do, they must be submitted to the FDA for their regulatory approval," Fauci said of the so-called mix-and-match trials. "So you don't want to get ahead of the FDA, but at least that's where the data are right now."
Data on Johnson & Johnson's mix-and-match study could be ready any day now, while Pfizer's trial might be completed by mid-October. Moderna's mix-and-match study data is already available, Fauci added.
If I got the Moderna or J&J vaccine should I get a booster shot of the Pfizer vaccine?
"That's a question that's been looked at to some extent in the U.K.," Boston Medical Center's Dr. Davidson Hamer recently told NBC10 Boston. "AstraZeneca was followed by Pfizer and has shown fairly robust immune response. The vaccine works in slightly different ways. We need more evidence, although the initial evidence shows that approach is safe."
For those who received the Moderna or Pfizer vaccine, the CDC says "a third dose of the same mRNA vaccine should be used."
"A person should not receive more than three mRNA vaccine doses. If the mRNA vaccine product given for the first two doses is not available or is unknown, either mRNA COVID-19 vaccine product may be administered," the agency's website states.
Fauci said last week that safety and efficacy data on mixing and matching COVID vaccines with boosters could be available within the next two weeks.
"As with all things we do, they must be submitted to the FDA for their regulatory approval," Fauci said of the so-called mix-and-match trials. "So you don't want to get ahead of the FDA, but at least that's where the data are right now."
But data on Johnson & Johnson's mix-and-match study could be ready soon, while Pfizer's trial might be completed by mid-October. Moderna's mix-and-match study data is already available, Fauci added.
Are there any risks with getting a booster shot? What about side effects?
Among people who stand to benefit from a booster, there are few risks, the CDC concluded. Serious side effects from the first two Pfizer doses are exceedingly rare, including heart inflammation that sometimes occurs in younger men. Data from Israel, which has given nearly 3 million people — mostly 60 and older — a third Pfizer dose, has uncovered no red flags.
The CDC has noted that side effects with the third shot "were similar to that of the two-dose series."
The most common symptoms include fatigue and pain at the injection site, but "most symptoms were mild to moderate."
As with previous doses of the vaccine, the CDC notes that, "serious side effects are rare, but may occur."
Hamer stressed that booster shots are safe, effective, and are unlikely to result in side effects like the initial doses.
"We've now vaccinated hundreds of millions with messenger RNA vaccines. We have a lot of safety data collected," Hamer said. "Giving a booster for most vaccines is unlikely to result in new side effects being identified... I don't anticipate any safety issues coming out as we start giving boosters."
Will booster shots contain the original vaccine, or one tailored to delta?
The boosters will be an extra dose of the original vaccine. Manufacturers still are studying experimental doses tweaked to better match delta. There’s no public data yet that it’s time to make such a dramatic switch, which would take more time to roll out. And independent research, including studies from Ellebedy’s team, shows the original vaccine produces antibodies that can target delta.
“I’m very, very confident that this vaccine will work against delta with a single booster of the same vaccine,” Pfizer CEO Albert Bourla told The Associated Press.
How much protection will I get?
No one yet knows “the magic line” — the antibody level known as the correlate of protection below which people are at risk for even mild infection, said immunologist Ali Ellebedy of Washington University at St. Louis.
But vaccines’ main purpose is to prevent severe disease. “It’s a very high bar to really go and say we can completely block infection,” Ellebedy noted.
Plus, people’s responses to their initial vaccination vary. Younger people, for example, tend to produce more antibodies to begin with than older adults. That means months later when antibody levels have naturally declined, some people may still have enough to fend off infection while others don’t.
CDC data show the vaccines still offer strong protection against serious illness for all ages, but there is a slight drop among the oldest adults. And immunity against milder infection appears to be waning months after people's initial immunization.
For most people, if you’re not in a group recommended for a booster, “it’s really because we think you’re well-protected,” said Dr. Matthew Daley of Kaiser Permanente Colorado.
Won't antibodies wane again even after a booster?
Eventually. “We don’t know the duration of protection following the boosters,” cautioned Dr. William Moss of Johns Hopkins University.
But antibodies are only one defense. If an infection sneaks past, white blood cells called T cells help prevent serious illness by killing virus-infected cells. Another type called memory B cells jump into action to make lots of new antibodies.
Those back-up systems help explain why protection against severe COVID-19 is holding strong so far for most people. One hint of trouble: CDC has preliminary data that effectiveness against hospitalization in people 75 and older dropped slightly in July -- to 80% -- compared to 94% or higher for other adults.
Am I still considered fully vaccinated if I don't get a booster?
Even with the introduction of boosters, someone who has gotten just the first two doses would still be considered fully vaccinated, according to the CDC's Dr. Kathleen Dooling. That is an important question to people in parts of the country where you need to show proof of vaccination to eat in a restaurant or enter other places of business.
The U.S. Centers for Disease Control and Prevention says you're fully vaccinated two weeks after receiving a second dose of the Pfizer or Moderna vaccine, or one dose of the J&J.