Fully Automated BioDetection
The BioFire FilmArray System is able to identify, in a closed system, dozens of the most lethal viruses and bacteria, including emerging infectious diseases. The easy-to-use system represents the next generation in automated detection systems.
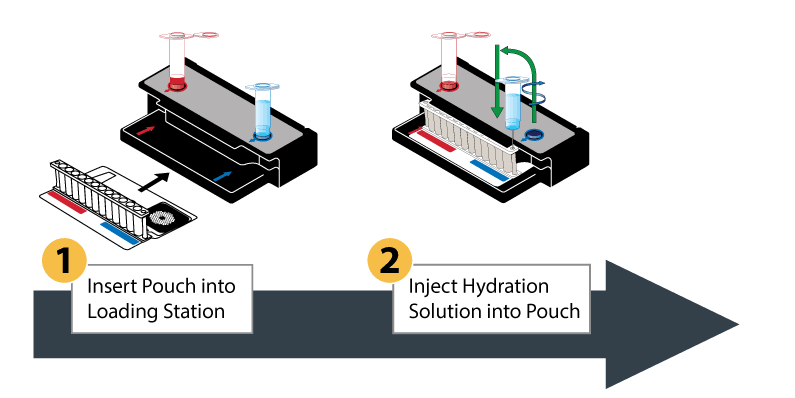
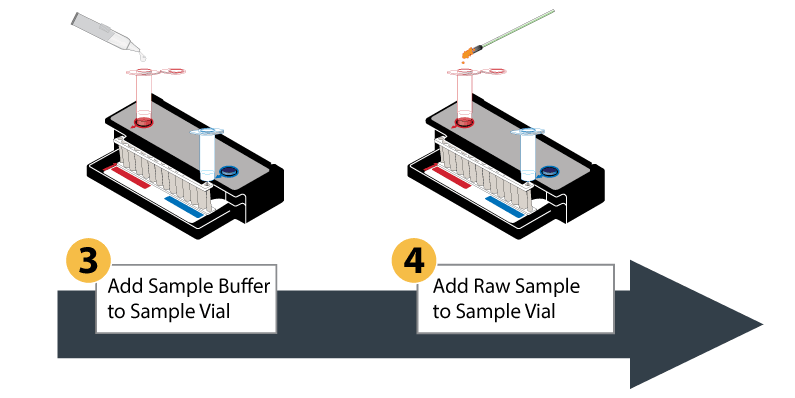
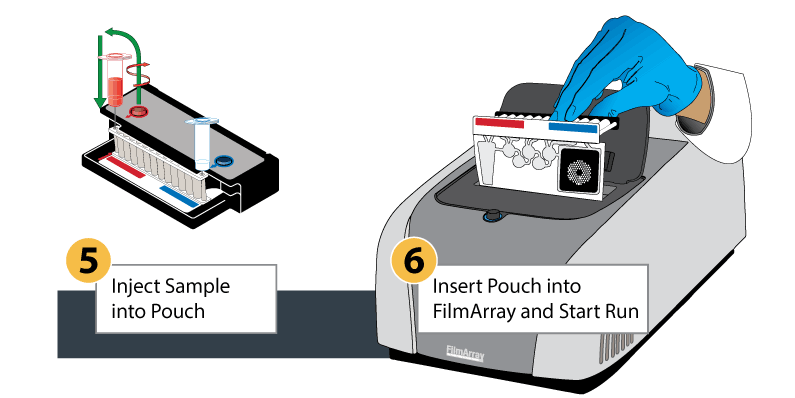
The BioFire FilmArray System uses a plastic pouch with automated capabilities, including sample preparation, reverse transcription for RNA viruses, and a two-stage nested multiplex PCR process. The results are a revolutionary detection system in a lightweight, small-footprint format.